
The OptiSafe Eye Irritation Test is a shelf-stable, test tube-based test that can be used to discriminate ocular irritants/corrosives from nonirritants and does not use live animal tissues or cells.
BACKGROUND
Ocular irritancy testing plays an important role in the labeling of chemicals and products to protect consumers and manufacturers from exposure to toxic chemicals through appropriate product and chemical labeling. In the past, the appropriate labeling of chemicals and compounds has relied on the in vivo Draize rabbit eye test, which has become the "gold standard" for chemical classification. This live animal test uses a clinical scoring system that grades the severity and duration of the ocular irritation response that generally occurs from 1 to 21 days after a chemicals exposure. The resulting clinical scores and duration of effects are then applied to either the United Nations Globally Harmonized System (GHS) or U.S. Environmental Protection Agency (EPA) methods of eye irritation classification. Because of animal cruelty concerns, there has been a major effort over the past several decades to develop alternative strategies (non-live animal tests) that could be safely used to classify and label potentially harmful chemicals. READ MORE
To address the need for a better and more predictive nonanimal ocular irritation test, we have been developing and improving the nonanimal ocular irritation test, OptiSafe.
INTRODUCTION TO OPTISAFE
Lebrun Labs LLC has been developing and improving the OptiSafe Eye Irritation Test method to serve as an accurate, reliable way to test consumer products and chemicals for their irritancy potential, without using animals.
OptiSafe Eye Irritation Test research was funded in 2015 by a National Institute of Environmental Health Sciences (NIEHS) phase I Research grant. Subsequently, the technology was patented, a three-lab validation study was performed, and Lebrun Labs LLC as awarded a phase II Small Business Innovation Research (SBIR) grant:
Lebrun Labs LLC Awarded SBIR phase I Grant to Develop a Nonanimal Test for Ocular Nonirritants
March 2015
GRANT NUMBER: R43ES025501
GRANT TITLE: Nonanimal Test Method to Determine The Ocular Safety Of Consumer Products and Chemicals
Award Info: NIEHS SBIR Grant. Research was supported by the NIEHS of the National Institutes of Health under Award Number R43ES025501. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Lebrun Labs LLC Awarded Patent on the OptiSafe Eye Irritation Test Method
August 7, 2018
Biochemistry-based ocular toxicity assay; United States Patent US10041922
Lebrun Labs LLC Awarded SBIR phase II Grant to Further Develop and commercialize the OptiSafe Nonanimal eye irritation test
December 2018
GRANT NUMBER: R44ES025501
GRANT TITLE: Nonanimal Test Method to Determine the Ocular Safety of Consumer Products and Chemicals: Phase II
Award Info: NIEHS SBIR Grant. Research was supported by the NIEHS of the National Institutes of Health under Award Number R44ES025501. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
OVERVIEW OF THE PROTOCOL
The OptiSafe Eye Irritation Test method allows users to start with any unknown test substance, including unground solids and intensely colored substances, and determine the irritancy classification based on specific biochemical assays. To both ensure the highest accuracy and streamline testing, pretests are performed. Pretest results are then applied to a decision tree that indicates which main test procedures should be performed.
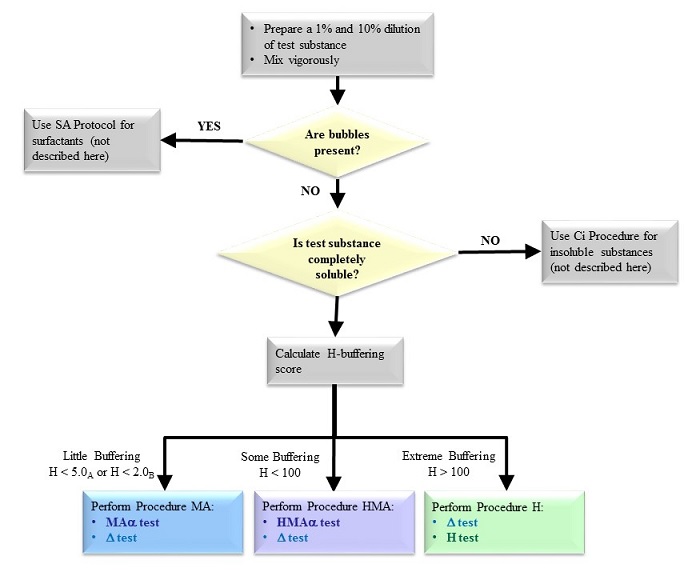 Figure 1. The pretest flowchart.
Figure 1. The pretest flowchart.
The pretest procedure is used to objectively assign the primary procedure that corresponds to the chemical and physical properties of the substance to be tested. The main procedures are inclusive to most materials including surfactants, nonsurfactants, and insoluble substances. Depending on the pretest outcome, one or two procedures are conducted:
-
Procedure α – assess denaturation
1. MAα – nonsurfactants with limited or little buffering capacity
2. HMAα – nonsurfactants with moderate buffering capacity
3. Ci – insoluble materials - Procedure Δ – assess water insoluble denaturation
- Procedure Η – assess ocular pH shift
Main Test Procedures
The main test procedure, selected using the pretest procedures, model denaturation and damage to biological molecules and tissues.
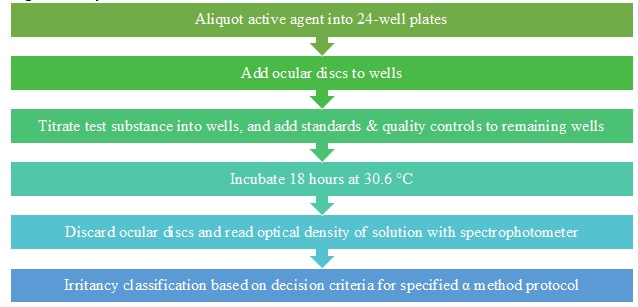 Figure 2. Overview of the alpha procedure.
Figure 2. Overview of the alpha procedure.
As shown in Figure 2, the active agent (test matrix) is aliquoted into a 24-well plate, discs are added to each experimental well, and the test substance, standards, and controls are titrated into the wells of the ocular membrane discs. After 18 hours of incubation, optical density (OD) values are measured using a spectrophotometer. An increase in the OD value indicates an increase in damage, indicating denaturation and reactivity of the test substance with the active agent. Following OD measurements, unknown values are compared to a standard curve to generate the irritation score. The irritation score is then applied to a prediction model to classify the unknown test material.
VALIDATION
The test method was validated in collaboration with NICEATM, ICCVAM, and the NTP. The NTP provided coded vials of the selected test chemicals to the lead lab and two naïve laboratories (the three-lab validation study). Interlaboratory reproducibility, accuracy, and false-negative rates were 91%, 89%, and 0%, respectively, for both the EPA and GHS classification systems. The false-positive rates were 23% for the GHS classification system and 25% for the EPA classification system. The success of these accuracy and transferability studies were further emphasized by the findings that there was low variation in irritation scores with similar final classifications for EPA category IV versus the rest or GHS not classified (NC) versus the rest, with transferability greater than 90%. These findings indicate that the OptiSafe test method is reproducible by naïve laboratories and demonstrates reliability in the manufacturing processes with little or no lot-to-lot variability, as shown by the low variation of irritation scores and the lack of any statistically different irritation scores between three different lots of OptiSafe test kits.
Overall, the OptiSafe Eye Irritation Test method performed better than all other test methods to which it was compared with differences in performance attributable to the superior sensitivity of the OptiSafe test method. This study’s data are consistent with the initial prevalidation data submitted to NICEATM and reviewed by ICCVAM, in which it was concluded that the OptiSafe test method compares favorably with other nonanimal test methods.
TECHNICAL SUMMARY
Accuracy is the number of correctly predicted chemicals out of all chemicals tested.
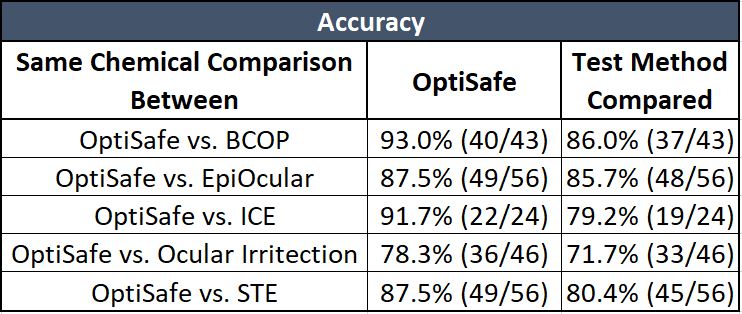
As shown on the table above, OptiSafe has the highest comparative accuracy.
Sensitivity is the number of correctly predicted positive (irritant) chemicals.
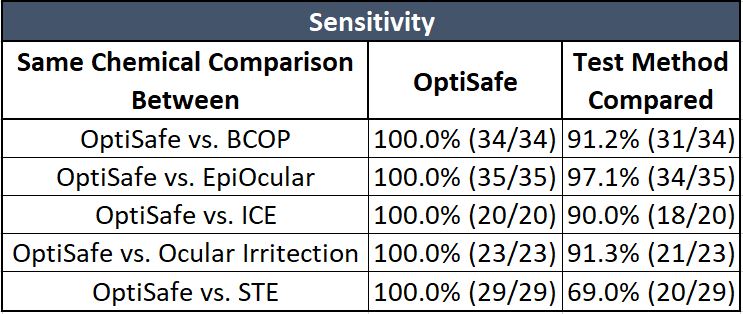
As shown on the table above, OptiSafe has a very high sensitivity.
Specificity is the number of correctly predicted negative chemicals.
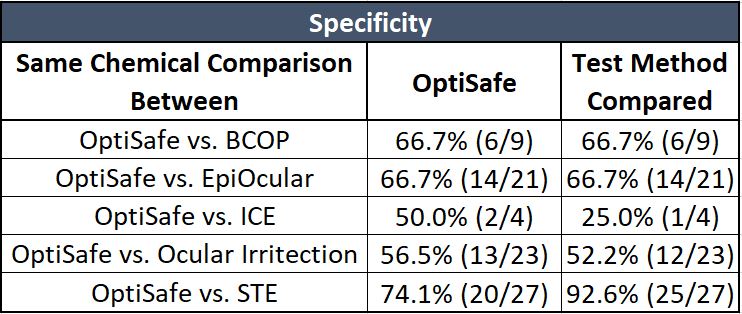
As shown on the table above, OptiSafe has a comparable specificity with other test methods with the exception of the STE method, which has a much lower sensitivity (see above table).
The false-negative rate is the number of true positives incorrectly predicted as negatives.
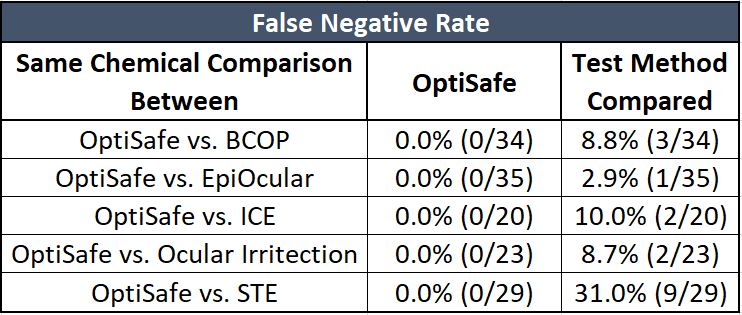
As shown on the table above, OptiSafe has the lowest false-negative rate (zero) of all the test methods evaluated.
The false-positive rate is the number of true negatives incorrectly predicted as positives.
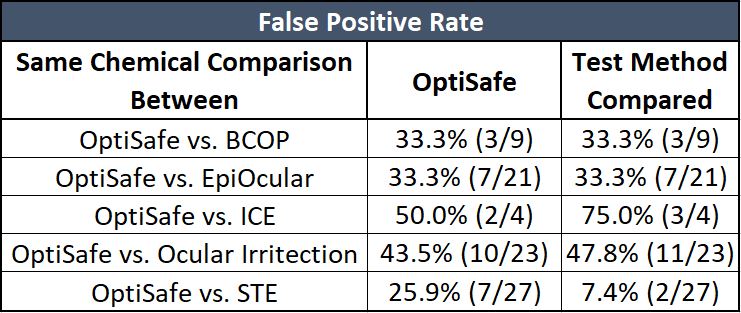
OptiSafe has a comparable false-positive rate except for the comparison with the STE method. However, the STE method has a high false-negative rate (see above table).
Overall, OptiSafe has a comparable specificity and false-positive rate and a better accuracy and much better sensitivity (100%) and false-negative rates (0). Most remarkable is the finding that there were no OptiSafe false negatives. In addition to better accuracy and very high sensitivity, other features of the test include: does not use or harm animals, does not require aseptic technique, has a rapid turn-around-time, and is economical and shelf stable. OptiSafe is a good first choice for the initial eye safety evaluation of personal care products, makeup, and a wide range of consumer products and chemicals.
TEST KITS AND SERVICES
We welcome interested parties and stakeholders to partner and collaborate with us as well as to use the OptiSafe Eye Irritation Test method. The test method is available as both a service and a test kit:
Kits are available from
Lebrun Labs LLC
Phone (714) 345-4689
sales@lebrunlabs.com